How should I do to make links to websites "clickable" when I send an e-mail?
If I use Explorer the links in e-mails always are blue and "clickable", I just click once on the link. When I use Firefox the links are not "clickable", I have to copy and paste the link directly into the browser. I am using Hotmail for my e-mails.
All Replies (4)
In incoming messages that you receive, it may be up to the sender whether the links are clickable. You could try an add-on to make text links clickable. In a search I found Linkification, but the official version has not yet been updated for Firefox 4 (there is an unofficial release at the author's web site).
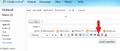
In outgoing messages that you compose, you should be able to use the link button (near the other formatting buttons) to create a clickable link.
I am not sure what the "link button" is. Do I find it in Firefox or in Hotmail?
One interesting thing: If I use my old e-mail client (FirstClass) the links always are clickable, even with Firefox. That may indicate that my problem is more linked with Hotmail than Firefox!
The link button is the one with the little piece of chain on it.
Thank you very much, my problem is solved!